In our animal vaccine business, we focus on developing products primarily for mammals, birds, and fish. We have experience with two formulation types—oral and injectable—and two product categories: feed additives and vaccines.
PCV2 Oral Vaccine / Feed Additives
Background
Porcine Circovirus Type 2 (PCV2) is the causative agent of Porcine Circovirus Associated Diseases (PCVAD), a widespread swine infection both in Japan and internationally. Infection is observed in nearly 100% of farms, leading to post-weaning multisystemic wasting syndrome (PMWS), which causes growth retardation, wasting, and reproductive disorders. The economic losses from PCVAD are estimated at up to 600 million euros annually in the EU.
PCV2 is a ubiquitous virus, highly resistant to disinfection and heat, making eradication extremely difficult. As a result, vaccination has become the standard control method. However, all commercial vaccines are injectable. In Japan, mass vaccination of all pigs is mandatory, yet this presents challenges such as labor and cost for individual injections, as well as the risk of needle fragments remaining in meat products.

Development Status
At KAICO, leveraging our proprietary silkworm–baculovirus expression system, we have developed oral vaccines that can induce antibody responses via oral administration (patents JP7678480, JP7502739).
In validation studies with pigs, we confirmed that silkworm powder is well accepted by animals, showing no palatability issues. When administered to piglets, the oral vaccine significantly reduced viremia and growth retardation, even under artificial viral exposure. Moreover, when administered to sows, antibodies were transferred to piglets through breast milk, demonstrating an additional pathway for effective immune protection. We continue to explore more efficient strategies for immunity transfer.
In parallel with pharmaceutical development, KAICO has also developed an immune-support feed additive designed to mitigate PCV2-related economic losses in the swine industry. The additive can be mixed with standard feed, stored at room temperature, and administered without injections. Product registration was completed in Vietnam in September 2024.
In a large-scale field trial involving approximately 2,000 pigs in Vietnam, the feed additive demonstrated equivalent growth performance compared to injectable vaccines while reducing labor time associated with vaccination by 99%.

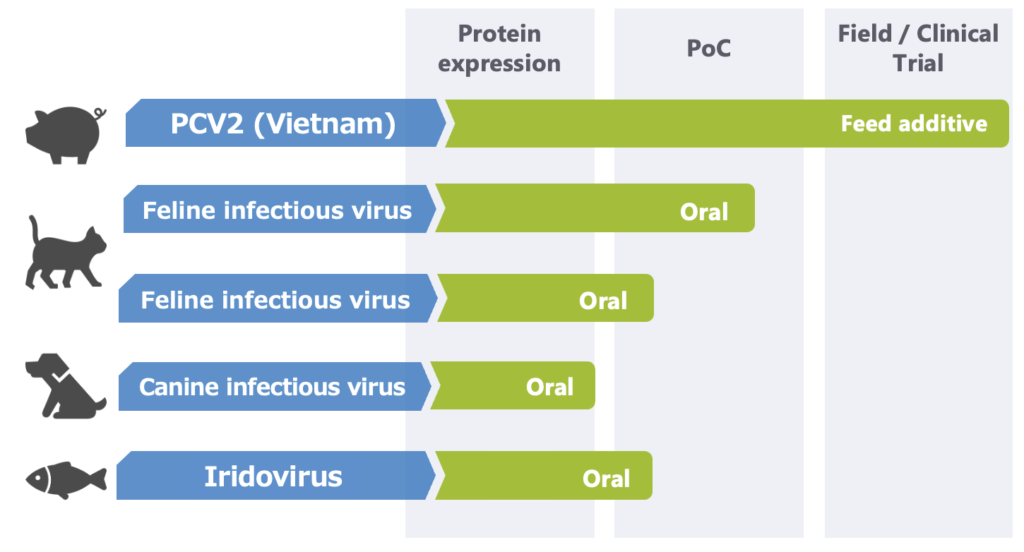
Animal Vaccine Development Pipeline
Through our silkworm–baculovirus expression platform, KAICO is advancing research on diverse antigens and oral vaccine formulations. Furthermore, by fostering alliances and joint research with a wide range of partners, we aim to create novel vaccine solutions together with domestic and international collaborators.
Protein Expression Services
KAICO provides protein expression services using our original silkworm-baculovirus system to supply proteins essential for vaccine development to manufacturers.
The silkworm-baculovirus expression system, which utilizes live silkworms, excels in expressing virus-like particles (VLPs) such as those used in our PCV2 vaccine. Additionally, we have successfully expressed the spike protein of SARS-CoV-2.



